- 16.01.2020 Valerian pill extract - a novelty in Ternopharm's product portfolio!
- 27.06.2019 Results of the conference of the Cherkassy Regional Medical Society of physician-otorhinolaryngologists
- 03.06.2019 On May 25, the official opening of the "Krajina Zdorov'ya" (Country of Health) Medical Center in the city of Vasilkov took place
- 03.06.2019 Meet the powerful novelty in the Panthenol family - Panthenol Plus cream from Ternopharm!
- 27.03.2019 Please welcome a Micropharm company recent development – Menovazin-solution with an automatic spray tube!
- 03.09.2018 New from Ternofarm!
Drug production of "Micropharm" llc is certified for the complience with GMP
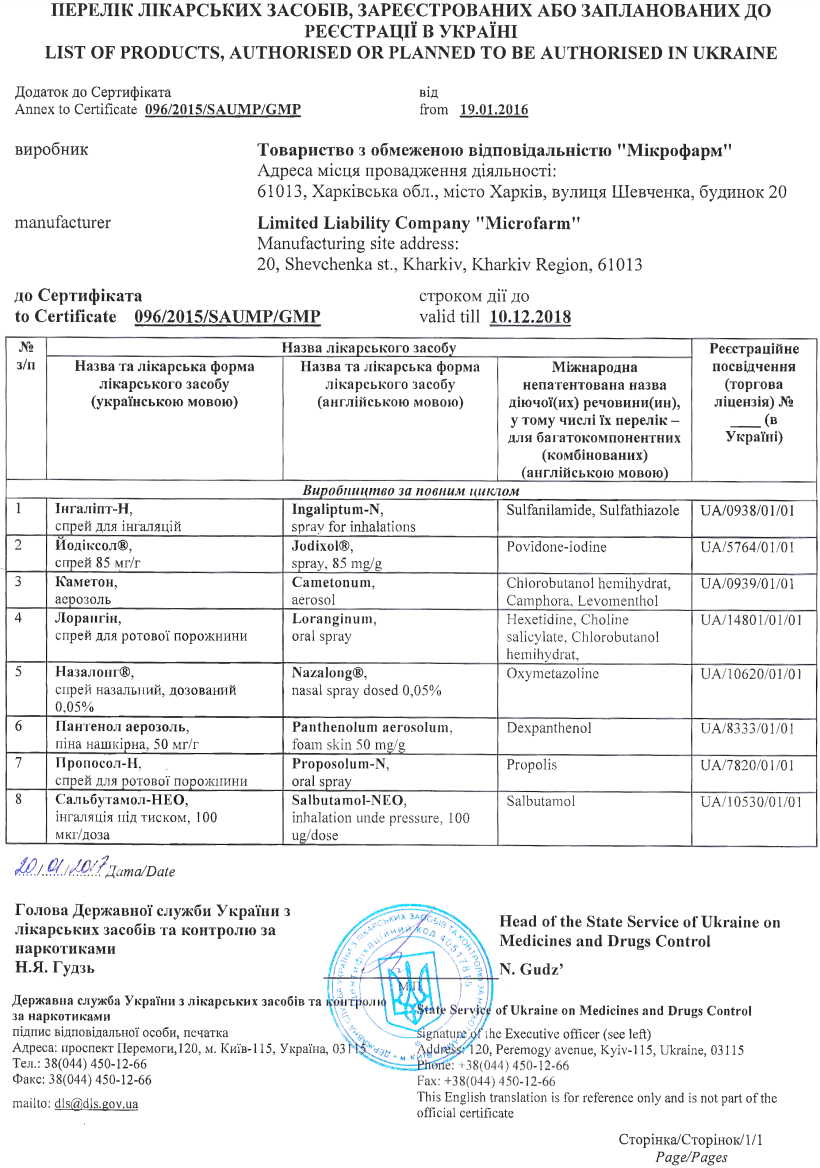
On December 7 – 10, 2015 the State Administration of Ukraine on Medical Products carried out the inspection
at the «Micropharm» for compliance with the GMP o the following production sites:
- production of finished medical products in the form of aerosols;
- production of finished medical products in the form of sprays
Based on the results of the inspection on January 19, 2016, to «Micropharm» LLC was issued certificate №096/2015/SAUMP/GMP, which testifies the compliance of medical products conditions with the requirements of Good Medical Practice.
According to the supplement for certificate on the manufacture of products complies with GMP requirements:
- Inhalipt-N
- Iodixol
- Cametonum
- Lorangin
- Nazalongum
- Panthenolum aerosolum
- Proposolum-N
- Salbutamol - NEO
GMP for "Micropharm" LLC means:
- rise of investor appeal
- opportunity to enter with their products to foreign markets
- rise of the products competitiveness
For "Unipharma" Holding, which includes the "Micropharm" Company,
this is the second GMP certificate and more confidence that the people of Ukraine will be provided with safe and effective medical products of good quality.